Trade Compliance
Trade
Compliance
Ensuring compliance with local and global trade regulations is essential for manufacturers, suppliers, and all supply chain stakeholders. Non-compliance poses significant risks beyond delivery delays, including stop-shipments, hefty penalties, and damaged brand reputation.
Our Trade Compliance Services offer comprehensive support, aiding clients in establishing robust import and export trade compliance programs. We navigate various US regulatory bodies such as ITA’s Import Administration, Export Administration Regulations (EAR), Export Control Reform Act (ECRA), International Traffic in Arms Regulations (ITAR), Customs-Trade Partnership Against Terrorism (C-TPAT), and more. Additionally, we provide guidance on HS/HTS codes, ECCN, EAR99 classification, CCL, NLR, Country of Origin, and related import/export information.

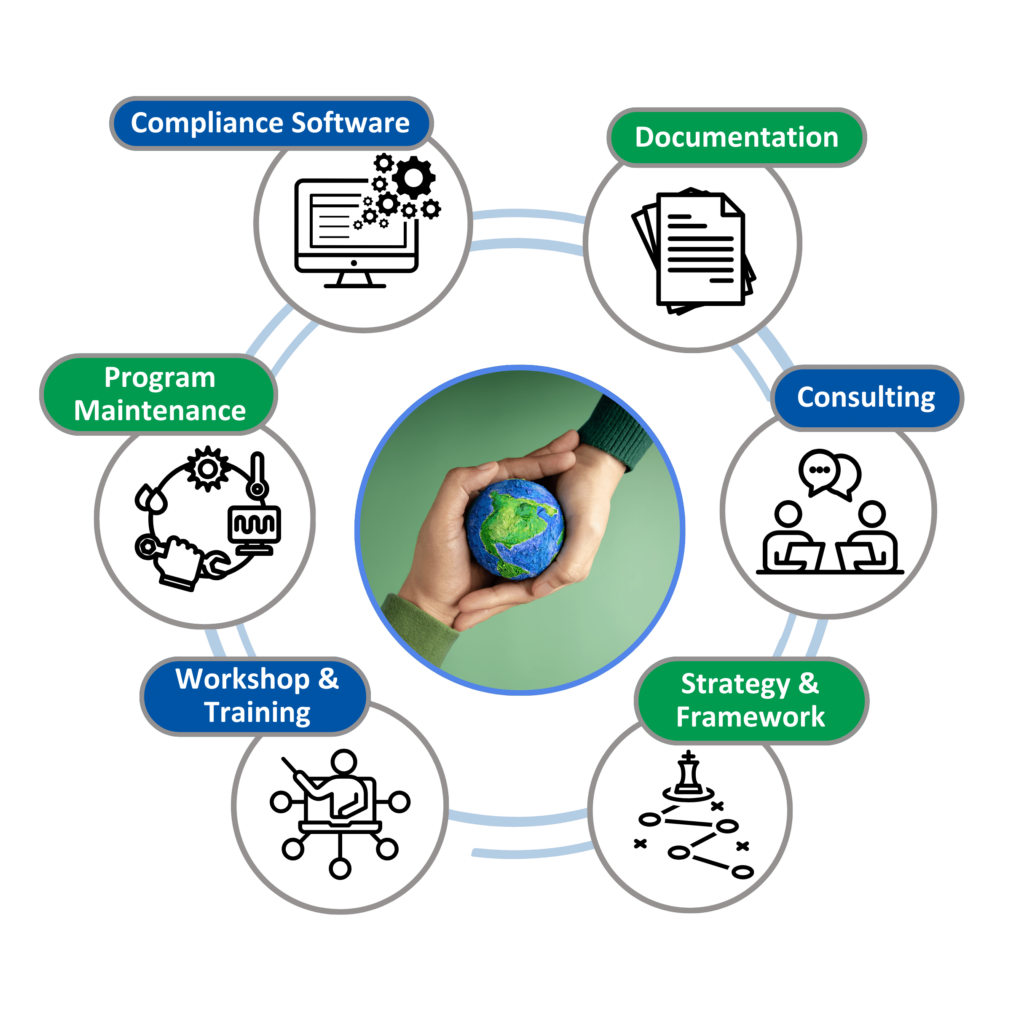
Solutions for Trade Compliance
Effective risk assessment is paramount as violations of trade compliance regulations can result in varying penalties based on factors such as the nature of goods, their origin and destination, and potential involvement in bribery. Continuous compliance training and awareness are imperative for companies. We facilitate ethical sourcing by enhancing visibility into supplier codes of conduct, gathering pertinent information on Child Labor, Anti-Human Trafficking, Supplier Diversity, workplace safety, and other ethical sourcing practices.
Our Trade Compliance solution offer consulting, training, and documentation to ensure your organization complies with relevant trade regulations, fostering sustainable growth and longevity while mitigating risks associated with market expansion and resource access.
Some of the areas of focus for trade compliance in Import/Export are
- Harmonized Tariff Schedule of the United States (HTSUS, known as HTS Coding)
- Harmonized Commodity Description and Coding System (HS coding)
- International Traffic in Arms Regulations (ITAR)
- Export Administration Regulations (EAR)
- International Trade Commission (ITC)
- International Trade Administration (ITA)
- Export Control Reform Act (ECRA)
- Importer of Record (IOR)


Services Portfolio
- Consulting: Assess compliance requirements, Gap assessments and identify opportunities for improvements
- Training: Training for internal and external stakeholders on trade compliance to make them aware of the implications of various applicable regulations.
- Supplier Engagement & Documentation: Collect, review, and validate compliance documents/declarations from your suppliers across the globe, to ensure they are meeting your trade compliance guidelines.